Fintech PR
Two-Thirds of Businesses Blame GenAI for Fraud Surge

- 62% of businesses cite generative AI as a key driver behind the surge in invoice fraud
- 7 out of 10 organizations were victims of fraud in 2024
- 90% lack experience in fraud prevention teams
- The perfect storm of challenges: new survey identifies company exposure to invoice fraud and lack of protection measures, causing global losses of $485bn
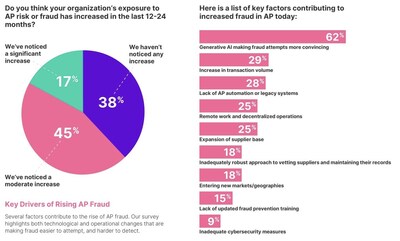
CHARLOTTE, N.C., Dec. 18, 2024 /PRNewswire/ — Two-thirds of businesses (62%) cite generative AI as a key driver behind the surge in invoice fraud, according to a new global survey. This increases difficulty in distinguishing between legitimate and fraudulent documents, as attacks grow in volume and sophistication.
Alongside this issue, 90% of organizations lack dedicated fraud prevention teams, forcing accounting staff to juggle fraud management beside their regular responsibilities, often straining resources and increasing vulnerability.
The findings were revealed in invoice automation company Basware’s ‘The Rise in AP Fraud‘ report, conducted by sharedserviceslink. The study surveyed 100 global business leaders and CFOs on the exposure companies have to financial fraud and what protection measures they have in place.
Fraud is a global $485bn issue
Fraud is becoming increasingly frequent, sophisticated, and damaging for businesses. In 2024, seven out of 10 organizations (68%) reported fraud attempts, with 62% noting these attempts had worsened over the past year.
The financial impact is staggering. Last year, fraud schemes, including bank fraud, resulted in global losses of $485 billion.
Remote work and decentralized operations are seen as key contributors of this surge, as identified by a quarter (25%) of respondents. By weakening internal controls, remote work exposes accounts payable (AP) departments to greater vulnerability, making it harder to prevent fraudulent activities.
At the same time, roughly a quarter of respondents also point to rising transaction volumes (29%) and the expansion of the supplier base (25%) as critical risk factors. With more invoices to process and a larger number of suppliers to manage, AP teams face greater complexity, which increases the likelihood of errors or fraud.
Together, these external threats create a perfect storm of challenges, making it easier for fraudsters to exploit weaknesses in the AP workflow.
GenAI used to create millions of illegitimate invoices
AP departments are on the frontlines of fraud detection, while also managing supplier payments, leaving them overwhelmed by the complexity and growing volume of fraudulent attempts.
Increasingly sophisticated tactics, such as fake invoices, deepfake media, and phishing scams – driven by generative AI – have made attacks more convincing and harder to detect. As a result, two-thirds (62%) of businesses now cite generative AI as a key driver behind the surge in AP fraud.
Tools such as ChatGPT enable the mass creation of communications and fake invoices, increasing risks and delays in resolving legitimate disputes. When handled by multiple disconnected systems, information becomes fragmented, making patterns harder to spot and allowing fraud to slip through undetected.
A high-impact case of AP fraud involved a man from Lithuania, who defrauded Facebook and Google out of more than $120 million by posing as a legitimate supplier. Using fake invoices, he secured $99 million from Facebook and $23 million from Google. The fraud underscored how vendor impersonation and fake invoices can cause massive financial losses without robust AP safeguards, even for large enterprises.
According to the survey, 90% of organizations lack dedicated fraud prevention teams, causing understaffed and under-resourced AP teams to prioritize speed over detailed checks, creating vulnerabilities. Meanwhile, reliance on paper-based processes and inadequate statement reconciliation leaves teams exposed to errors, inefficiencies, and fraudulent activities.
Tom Santacroce Global VP of AP Assurance, commented on the findings:
“Manual processes are inherently slow and prone to errors, making it difficult to match invoices, track approvals, or identify duplicate payments – creating exploitable gaps for fraudsters, who are now using GenAI. For overburdened AP teams, these create the perfect storm of challenges, leading to operational bottlenecks, strained supplier relationships, and lost cash flow.
“Forward-thinking organizations are reimagining fraud, overpayment and risk prevention through AI and automation that protects against increasingly complex financial threats. Remote work has weakened traditional security, requiring secure systems and innovative solutions like decentralized finance and blockchain for transparent vendor transactions. With increasingly sophisticated fraud tactics on the uptick, organizations must prepare for stricter AI and compliance rules. Proactive fraud prevention today not only mitigates risk, but also future-proofs operations against costly penalties.”
How enterprises can combat fraud
AP fraud is one of the most targeted types of fraud for businesses. According to the survey, 28% of organizations cite a lack of AP automation to help tackle fraud. Reliance on manual processes and outdated tools leaves businesses ill-equipped to manage complex, high-volume transactions, creating critical gaps for fraudsters to exploit.
One example of how automation has strengthened AP fraud prevention is KION, a global leader in forklift trucks and warehouse equipment, who were previously vulnerable to fraud risks due to manual invoice processing.
To address these challenges, KION partnered with Basware, shifting to automated AP processes driven by AI that significantly reduced manual errors, minimizing fraud opportunities. With over 90% of its spend now controlled, KION has streamlined its operations, providing better visibility, control, and oversight — ensuring that invoices and payments are accurately validated, reducing the risk of errors or AP fraud.
For the full report, visit: https://www.basware.com/en/resources/the-rise-in-ap-fraud-frequency-sophistication-and-impact
About Basware
Basware is how finance leaders in global enterprises can finally automate their complex, labor-intensive invoice processes and stay compliant with regulatory change. Our AP automation and invoicing platform helps you achieve a new level of efficiency – in a matter of months – while reducing errors and risks. We bring a unique combination of true automation, complete coverage, and deeper expertise to make it all just happen for our customers. That’s why the world’s most efficient AP departments at thousands of companies rely on Basware to handle over 220 million invoices per year. With Basware, Now it all just happens.™
Photo – https://mma.prnewswire.com/media/2583049/Rise_in_AP_Fraud.jpg
Photo – https://mma.prnewswire.com/media/2583047/Key_Factors.jpg
Photo – https://mma.prnewswire.com/media/2583048/Who_primarily_manages.jpg
Logo – https://mma.prnewswire.com/media/2398888/Basware_logo.jpg


![]() View original content:https://www.prnewswire.co.uk/news-releases/two-thirds-of-businesses-blame-genai-for-fraud-surge-302334046.html
View original content:https://www.prnewswire.co.uk/news-releases/two-thirds-of-businesses-blame-genai-for-fraud-surge-302334046.html

Fintech PR
NYSE CONTENT ADVISORY: TODAY’S PRE-MARKET UPDATE DECEMBER 18, 2024
NEW YORK, Dec. 18, 2024 /PRNewswire/ — The New York Stock Exchange (NYSE) is proud to offer a new daily pre-market update and additional content directly from the iconic NYSE Trading Floor.
Access the new Daily NYSE Pre-market update and additional content here: https://www.multivu.com/nyse/9306251-en-new-york-stock-exchange-pre-market-update
DAILY NYSE PRE-MARKET UPDATE
Kristen Scholer, Senior Markets Anchor, NYSE, delivers a daily pre-market update that includes key insights into the trading day ahead leading up to the NYSE’s Opening Bell.
NYSE ORIGINAL CONTENT:
Elevate your reporting with the latest market insights and content from the NYSE, the world’s leading financial marketplace by leveraging a range of exclusive NYSE content including:
- NYSE Photo Highlights: NYSE-listed companies, Trading Floor moments, Leadership events.
- NYSE B-Roll Footage: NYSE Trading Floor, Market milestones, and Bell-ringing events.
- NYSE Original Content:
- Floor Talk: Exclusive interviews with industry trend-setters and innovators.
- Inside the ICE House Podcast: Conversations with CEO, founders, and leaders.
- Taking Stock: Go face-to-face with visionary entrepreneurs who are redefining sectors.

Video – https://mma.prnewswire.com/media/2584161/NYSE_Market_Dec_18_2024.mp4
Logo – https://mma.prnewswire.com/media/2581322/5084577/New_York_Stock_Exchange_Logo.jpg
![]() View original content:https://www.prnewswire.co.uk/news-releases/nyse-content-advisory-todays-pre-market-update-december-18-2024-302335036.html
View original content:https://www.prnewswire.co.uk/news-releases/nyse-content-advisory-todays-pre-market-update-december-18-2024-302335036.html

Fintech PR
Gan & Lee Pharmaceuticals Announces U.S. FDA Clearance of the IND application for the innovative Bi-weekly GLP-1RA GZR18 Injection, Bofanglutide, with chronic weight management Indication (A Phase 2 head-to-head with Tirzepatide clinical trial)
BEIJING and BRIDGEWATER, N.J., Dec. 18, 2024 /PRNewswire/ — Gan & Lee Pharmaceuticals (Gan & Lee, Shanghai Stock Exchange: 603087.SH), is pleased to announce that the Food and Drug Administration (” FDA “) has cleared the Investigational New Drug (IND) application for GZR18 Injection to conduct a phase 2 clinical trial, a head-to-head with Tirzepatide from Eli Lilly and Company (NYSE: LLY) in the US (NCT06737042). GZR18 is a long-acting glucagon-like peptide-1 (GLP-1) receptor agonist (RA) being developed by Gan & Lee Pharmaceuticals. The clinical trial indication of this application is the chronic weight management for obese or overweight patients, with or without T2DM.
According to the latest data released by the World Obesity Federation (WOF) Global Obesity Report (2024), approximately 2.2 billion adults worldwide were overweight (referring to BMI ≥ 25kg/m2) in 2020, accounting for about 42% of the total adult population. It is expected that this number will rise to 3.3 billion by 20351. Obesity can lead to a series of complications, including diabetes, cardiovascular diseases and even mental diseases such as depression. The medical expenses caused by obesity and its complications have brought a heavy medical burden to patients and society.
GZR18, as a GLP-1 receptor agonist, can delay gastric emptying by activating GLP-1 receptors expressed on the gastrointestinal tract; and enhance satiety and suppress appetite by activating GLP-1 receptors in the hypothalamus and other parts, thereby reducing the patient’s weight. GZR18 injection is the first bi–weekly GLP-1 mono-agonist formulation. Current clinical data has demonstrated weight loss effects comparable to or even better than multi-target once-weekly GLP-1 formulations, providing new insights for the future development of GLP-1 drugs. The development of the bi-weekly GZR18 injection is expected to offer more flexible treatment options for obese patients , leading to improved long-term weight management efficacy and adherence.
About GZR18
GZR18 is a long-acting glucagon-like peptide-1 (GLP-1) receptor agonist (RA) being developed by Gan & Lee Pharmaceuticals. The indications currently under development are type 2 diabetes and chronic weight management for obese or overweight patients. Clinical data shows that administering GZR18 injection once a week and every two weeks can achieve good hypoglycemic or weight loss effects2.
References:
1. World Obesity Alliance 2024 World Obesity Report [EB/OL]. London: World Obesity Alliance,
2. LINONG JI, WEI CHEN, RUIHUA DONG, MINGXIA YUAN, DONG ZHAO, SHUGUANG PANG, LIYUAN ZHAO, JING ZHAO, ZHONG-RU GAN; 1858-LB: A Novel GLP-1 Analog, GZR18, Induced an 18.6% Weight Reduction in Subjects with Obesity in a Phase Ib/IIa Trial. Diabetes 14 June 2024; 73 (Supplement_1): 1858–LB. https://doi.org/10.2337/db24-1858-LB
About Gan & Lee
Gan & Lee Pharmaceuticals developed the first Chinese domestic insulin analog. Currently, Gan & Lee has six core insulin products, including five insulin analog varieties: long-acting glargine injection (Basalin®), fast-acting lispro injection (Prandilin™), fast-acting aspart injection (Rapilin®), mixed protamine zinc lispro injection (25R) (Prandilin™25), aspart 30 injection (Rapilin®30), and one human insulin injection – mixed protamine human insulin injection (30R) (Similin®30). The company has two approved medical devices in China, namely reusable insulin injection pen (GanleePen), and disposable pen needle (GanleeFine®).
In China’s 2024 National Insulin-Specific Centralized Procurement, Gan & Lee Pharmaceuticals ranked first among all selected companies in terms of procurement demand for insulin analogs. The company is also making strides in international markets, with the disposable pen needle (GanleeFine®) approved by the US Food and Drug Administration (FDA) in 2020 and received GMP inspection approval from the European Medicines Agency (EMA) in 2024. These achievements significantly boost Gan & Lee’s competitiveness in both international and domestic markets.
In the future, Gan & Lee will strive for comprehensive coverage in diabetes treatment. Moving forward with its mission to become a world-class pharmaceutical company, Gan & Lee will also actively develop new chemical entities and biological drugs, focusing on treatments for metabolic diseases, cardiovascular diseases, and other therapeutic areas.
Further Information:
[email protected] (Media)
[email protected] (Business Development)
[email protected] (Medical Information)
Logo – https://mma.prnewswire.com/media/2439708/Gan_Lee_Pharmaceuticals_Logo.jpg

Fintech PR
Smartkem Receives £900,000 Grant from Innovate UK for Advanced MicroLED Displays
Project in partnership with AUO starts on January 1, 2025
MANCHESTER, England, Dec. 18, 2024 /PRNewswire/ — Smartkem (Nasdaq: SMTK), which is seeking to change the world of electronics using its disruptive organic thin-film transistors (OTFTs), has received and accepted a £900,000 (USD 1.1 million) grant from Innovate UK for its previously announced project partnership with AUO to develop a rollable, transparent microLED display. Part of the 2024 UK-Taiwan Collaborative R&D Initiative, the 2-year project will commence on January 1, 2025, with initial grant payments beginning in the first quarter of 2025.
About the 2024 UK-Taiwan Collaborative R&D Initiative
The 2024 UK-Taiwan Collaborative R&D Initiative has invested more than £10 million this year to promote bilateral industrial technology research and development cooperation. The nine award-winning projects will promote the joint development of advanced technologies in fields such as electrical information communication, biomedicine, and electromechanical by Taiwan-UK enterprises.
About Smartkem
Smartkem is seeking to reshape the world of electronics with its disruptive organic thin-film transistors (OTFTs) that have the potential to revolutionize the display industry. Smartkem’s patented TRUFLEX® liquid semiconductor polymers can be used to make a new type of transistor that can be used in a number of display technologies, including next generation microLED displays. Smartkem’s organic inks enable low temperature printing processes that are compatible with existing manufacturing infrastructure to deliver low-cost displays that outperform existing technology.
Smartkem develops its materials at its research and development facility in Manchester, UK and provides prototyping services at the Centre for Process Innovation (CPI) at Sedgefield, UK. It has a field application office in Taiwan. The company has an extensive IP portfolio including 138 granted patents across 18 patent families, 16 pending patents and 40 codified trade secrets. For more information, visit: www.Smartkem.com and follow us on LinkedIn http://www.linkedin.com/company/Smartkem-limited.
Forward-Looking Statements
All statements in this press release that are not historical are forward-looking statements, including, among other things, its market position and market opportunity, expectations and plans as to its product development, manufacturing and sales, and relations with its partners and investors. These statements are not historical facts but rather are based on Smartkem Inc.’s current expectations, estimates, and projections regarding its business, operations and other similar or related factors. Words such as “may,” “will,” “could,” “would,” “should,” “anticipate,” “predict,” “potential,” “continue,” “expect,” “intend,” “plan,” “project,” “believe,” “estimate,” and other similar or elated expressions are used to identify these forward-looking statements, although not all forward-looking statements contain these words. You should not place undue reliance on forward-looking statements because they involve known and unknown risks, uncertainties, and assumptions that are difficult or impossible to predict and, in some cases, beyond the Company’s control. Actual results may differ materially from those in the forward-looking statements as a result of a number of factors, including those described in the Company’s filings with the Securities and Exchange Commission. The Company undertakes no obligation to revise or update information in this release to reflect events or circumstances in the future, even if new information becomes available.
Contacts:
Selena Kirkwood
Head of Communications for Smartkem
T: +44 (0) 7971 460 364
[email protected]
U.S. Investors
David Barnard, CFA
Alliance Advisors Investor Relations
T: 1 415 433 3777
[email protected]
View original content:https://www.prnewswire.co.uk/news-releases/smartkem-receives-900-000-grant-from-innovate-uk-for-advanced-microled-displays-302334970.html

-

 Fintech7 days ago
Fintech7 days agoFintech Pulse: Your Daily Industry Brief (IBANera, FIS, Citigroup, Gen Digital, Mynt)
-

 Fintech6 days ago
Fintech6 days agoFintech Pulse: Your Daily Industry Brief (Nuvei, Google, Upvest, Gen Digital, MoneyLion)
-

 Fintech PR5 days ago
Fintech PR5 days agoCathay Financial Holdings Calls for Climate Finance Mobilization to Drive the Climate Industrial Revolution
-

 Fintech PR5 days ago
Fintech PR5 days agoA New Era of $WUSD — Revolutionizing Stablecoins with Unmatched Security, Stability and Next-Gen Innovation
-

 Fintech PR5 days ago
Fintech PR5 days agoLanistar launches new gaming sites in Brazil as secures right to operate pending final approval on its licence
-

 Fintech2 days ago
Fintech2 days agoFintech Pulse: Your Daily Industry Brief (Synapse, Shenzhen Institute, Visa, AutomatIQ, MeridianLink)
-

 Fintech PR5 days ago
Fintech PR5 days agoHealthcare Revenue Cycle Management (RCM) Market Surges to USD 658.7 Billion by 2030, Propelled by 24% CAGR – Verified Market Reports®
-

 Fintech PR5 days ago
Fintech PR5 days agoInternational Communication Forum: Pathways To A Sustainable Future




