Fintech PR
GemVax Announces Topline Results from Phase 2a Progressive Supranuclear Palsy Clinical Trial at Neuro2024

– Topline supports moving to Phase 3 trial and shows potential to develop GV1001 as the world’s first PSP treatment
SEOUL, South Korea, Oct. 29, 2024 /PRNewswire/ — GemVax & KAEL Co., Ltd. (“GemVax”; KOSDAQ ticker: 082270) announced that topline results of a Phase 2a clinical trial (the “Phase 2a PSP Clinical Trial”) of GV1001, an investigational peptide drug for the treatment of progressive supranuclear palsy (“PSP”), were presented at “Neuro2024: The PSP and CBD International Research Symposium” in Toronto, Canada, at 4:45 p.m. local time on 24th October.
PSP is a degenerative disease that, like Parkinson’s disease, causes symptoms such as gait disturbances, early falls, vertical gaze palsy, rigidity, tremors, and cognitive decline, but it progresses faster and currently has no fundamental treatment. PSP is classified into several types, including PSP-Richardson’s syndrome (“PSP-RS”) and PSP-parkinsonism (“PSP-P”). Compared to other types of PSP, the PSP-RS type shows a greater accumulation of tau protein and affects larger areas, including the cerebellum, dentate nucleus, pontine nuclei, frontal lobe, and parietal lobe.
The Phase 2a PSP Clinical Trial was a 24-week, randomized, double-blind, placebo-controlled, prospective exploratory clinical trial conducted in 78 patients with PSP at 5 centers in Korea. The participants were randomized 1:1:1 to receive either placebo or GV1001 0.56 mg or GV1001 1.12 mg administered subcutaneously once weekly for the first 4 weeks (1 month), and then at 2-week intervals for 20 weeks (5 months) for a total of 24 weeks (6 months). Patients with both PSP-RS and PSP-P types were eligible to participate in the study. Results showed higher benefits in the lower dose group (0.56 mg), particularly in PSP-RS type patients.
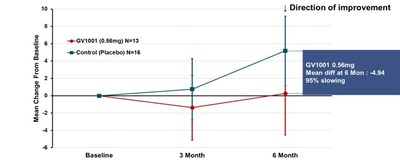
The primary endpoint of the trial was change from baseline in total score (calculated as the least-square mean using MMRM method) of PSP-Rating Scale after 24 weeks of GV1001 administration, which showed deterioration by 2.14 points in GV1001 0.56 mg dose group compared to 4.10 points in the placebo group, demonstrating a 48% reduction in disease progression (see Figure 1). Although statistical significance was not demonstrated, the results support the potential of GV1001 as a treatment of PSP, a disease for which there is currently no cure, and the potential to advance GV1001 into further clinical trials.
The clinically typical PSP is often referred to as the PSP-RS type, which accounts for the majority of PSP patients. This type progresses faster and has a shorter average survival time compared to other PSP types. Subgroup analysis was conducted in patients with PSP-RS type only. The change from baseline in PSP-Rating Scale total score mean (calculated using simple average) at 24 weeks of GV1001 administration to PSP-RS type patients was a deterioration by 0.25 points in the GV1001 0.56 mg dose group compared to a deterioration by 5.19 points in the placebo group, demonstrating a 4.94-point difference or a 95% reduction in disease progression (see Figure 2).
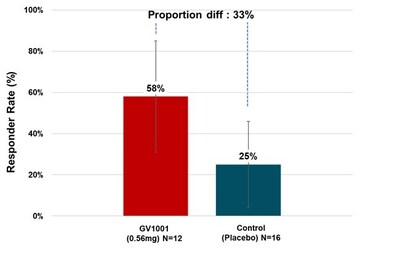
Many PSP-RS type patients in the treatment group experienced symptom stabilization or even improvement during the clinical period. When calculated as responder rate based on the percentage of patients whose PSP Rating Scale scores improved or remained stable after six months of treatment compared to baseline, 58.33% of PSP-RS type patients in the 0.56 mg GV1001 group showed improvement or stabilization (see Figure3).
The safety profile of GV1001 in the Phase 2a PSP Clinical Trial was consistent with prior safety data. GV1001 was generally well-tolerated with no serious adverse events related to the drug reported.
Hyungsik Moon, CSO of GemVax, stated that “this Phase 2a trial was an exploratory study to determine the optimal dosage and find out how the peptide works on different subgroups. Although the topline result did not achieve statistical significance, the evidence is strong enough to consider moving forward to a pivotal trial and shows potential to develop GV1001 as the world’s first treatment option for PSP.”
Experts at the Neuro2024 meeting welcomed the results of the PSP trial as encouraging and expressed excitement for the drug to enter a global Phase 3 clinical trial for further development.
“This pilot study was not fully powered and the treatment duration with 6 months was short. Thus, statistically significant confirmatory results could not be expected” said Peter Schüler, MD, Senior Vice President of Drug Development at global CRO ICON. “Nonetheless, the observed trends are very plausible and consistent in two domains: motor performance and cognitive function, both favoring the lower dose group.”
“The trial identified the optimal dose, which was one of the primary objectives of the Phase 2a study, and demonstrated clinically meaningful benefits, namely full stabilization of the disease compared to the placebo group,” said Dr. Schüler, adding “these topline results provide a strong foundation for advancing to Phase 3.”
Dr. Günter U. Höglinger, Head of the Department of Neurology, LMU Hospital, Munich, and a world-renowned expert in PSP, commented: “very exciting Phase 2 level data with novel drug study with new mechanisms of action. Data is preliminary but very promising and it is in line with [GV1001] Alzheimer’s disease clinical data. I look forward to further development and very excited to participate and lead the [PSP] Phase 3 study.”
Dr. Kristophe Diaz, Director of CurePSP, said that “we are encouraged by the results of the recent GemVax clinical trial, which offer hope to the entire PSP community, including patients who currently have no treatment options, their families and the physicians who care for them” and that “we congratulate GemVax on the successful completion of this trial and look forward to further developments that benefit the PSP community.” He also said that “CurePSP remains committed to collaborating and supporting efforts that bring hope and progress for those affected by this devastating disease.”
Download PDF: https://mma.prnewswire.com/media/2542940/2024_10_29__GemVax_Announces_Topline_Results.pdf
About Phase 2a PSP Clinical Trial (NCT05819658)
The Phase 2a PSP clinical trial was a 24-week, multicenter, randomized, double-blind, placebo-controlled, prospective phase 2a exploratory clinical trial to evaluate the safety and efficacy of GV1001 0.56 mg or 1.12 mg compared to placebo for the treatment of patients with PSP. The primary outcome of the study was change from baseline in the total score of PSP-Rating Scale after 24 weeks of GV1001 administration. Secondary endpoints included change from baseline in the total score of PSP-Rating Scale at 3 months, MoCA-K, K-FAB and ES-ADL at both 3 and 6 months. Overall safety of GV1001 administration was also assessed.
About GV1001
GV1001 is a synthetic peptide consisting of 16 amino acids based on the key sequence of telomerase. GV1001 has been studied for the potential treatment of neurodegenerative diseases including Alzheimer’s disease and PSP. In neurodegenerative diseases, GV1001 has been demonstrated to modulate phenotypes of glial cells, and to regulate neuroinflammation. In addition to the Phase 2a PSP clinical trial, a Phase 2 Alzheimer’s disease clinical trial of GV1001 is currently ongoing in the U.S. and Europe (NCT05189210).
About PSP
Progressive supranuclear palsy is a rare progressive and adult-onset neurodegenerative disease that currently has no disease-modifying drug. Approximately seven in 100,000 people worldwide is affected by PSP and is more common in men. People over the age of 60 are mainly affected. The symptoms of PSP include loss of balance, changes in personality, weakness of eye movements, especially in the downward direction, difficulty in swallowing, slurred speech and cognitive impairment.
About GemVax & KAEL
GemVax & KAEL Co., Ltd. is a pioneering clinical-stage biopharmaceutical company based in Korea, dedicated to developing proprietary therapeutics for neurodegenerative diseases including progressive supranuclear palsy and Alzheimer’s disease. As for PSP, GemVax is currently conducting a Phase 2a study in Korea to evaluate the efficacy and safety of GV1001 in patients with PSP. Preparations are also underway for a global PSP clinical trial. In addition, GemVax is currently conducting a Phase 2 Alzheimer’s disease clinical trial in the U.S. and Europe. For more information, visit www.gemvax.com and follow us on Linkedin.
Forward-Looking Statements
This document contains information that includes or is based upon “forward-looking statements” within the meaning of the Securities Litigation Reform Act of 1995. Forward-looking statements may or may not include identifying words such as “plan,” “will,” “expect,” “anticipate,” “intend,” “believe,” “potential,” “continue,” and similar terms. These statements are subject to known or unknown risks and uncertainties that could cause actual results to differ materially from those expressed or implied in such statements, including but not limited to: challenges inherent in pharmaceutical research and development, including the timing and results of preclinical and clinical programs, where the risk of failure is high and failure can occur at any stage prior to or after regulatory approval due to lack of sufficient efficacy, safety considerations, or other factors; our ability to leverage and enhance our drug discovery platform; our ability to obtain financing for development activities and other corporate purposes; the success of our collaboration activities; our ability to obtain regulatory approval of, and ultimately commercialize, drug candidates; our ability to obtain, maintain, and enforce intellectual property protections; cyberattacks or other disruptions to our technology systems; our ability to attract, motivate, and retain key employees and manage our growth; inflation and other macroeconomic issues; and other risks and uncertainties. All forward-looking statements are based on management’s current estimates, projections, and assumptions, and GemVax undertakes no obligation to correct or update any such statements, whether as a result of new information, future developments, or otherwise, except to the extent required by applicable law.
Photo – https://mma.prnewswire.com/media/2543033/Figure_1.jpg
Photo – https://mma.prnewswire.com/media/2543034/Figure_2.jpg
Photo – https://mma.prnewswire.com/media/2543035/Figure_3.jpg
PDF – https://mma.prnewswire.com/media/2543036/2024_10_29__GemVax_Announces_Topline_Results.pdf
Logo – https://mma.prnewswire.com/media/2542900/4994690/GemVax_Logo.jpg
![]() View original content to download multimedia:https://www.prnewswire.co.uk/news-releases/gemvax-announces-topline-results-from-phase-2a-progressive-supranuclear-palsy-clinical-trial-at-neuro2024-302289880.html
View original content to download multimedia:https://www.prnewswire.co.uk/news-releases/gemvax-announces-topline-results-from-phase-2a-progressive-supranuclear-palsy-clinical-trial-at-neuro2024-302289880.html

Fintech PR
Oxford don Levy’s VC-think-tank Blue Capital announces Hon. Hugh E. Powell as Partner

Powell is former Advisor to UK Prime Minister David Cameron.
SAN FRANCISCO, Jan. 6, 2025 /PRNewswire/ — Blue Capital, the VC-think-tank firm, has appointed The Honorable Hugh E. Powell as Partner. Powell has served as Advisor for more than a year, providing strategic guidance and now accepts this enhanced role.
“We are enthusiastic to welcome Hugh as Partner,” said Matthew Chase Levy, General Partner of Blue Capital. “His expertise and capabilities will be invaluable as we continue to identify and support the most promising efforts in the sector. Hugh is deeply aligned with our mission to accelerate the transition to a more powerful and secure energy future. I have personally learned a great deal from him during his time advising and look forward to our greater partnership.”
The Hon. Hugh E. Powell brings diplomatic acumen and strategic insight to Blue Capital. As the former Deputy National Security Advisor of the UK under Prime Minister Lord David Cameron, he possesses a deep understanding of the geopolitical landscape and the critical role of energy security in global stability. His experience in navigating complex international relations has been instrumental in guiding Blue Capital’s investment strategy and fostering partnerships across borders. Powell served in Helmand, Afghanistan, and previously in the embassies in Berlin and Paris. He also spent 4 years as an adviser to Macquarie Bank. “I am deeply honored to join Blue Capital as a Partner,” said Powell. “Matthew and the team have built something truly special – a firm with not only a clear vision for the future of energy but also the expertise and network to make that vision a reality.”
Blue Capital was founded by Matthew Chase Levy, Honorary Fellow of Wolfson College, Oxford, the youngest Don in the Fellowship’s history. Levy is the first American physicist to be Sir Isaac Newton Fellow in The Royal Society, an institution re-founded in 1662 by King Charles II.
“The appointment of Hugh as Partner has significantly strengthened Blue Capital’s capabilities. We are honored to have him and his support” said Levy. “Finally, I am grateful to Hon. Jo C. Bamford for his firm’s advice to consider to re-brand to Blue Capital. We admire its elegance.”
About Blue Capital:
Blue Capital is the VC-think-tank that invests in people working to defeat existential threats using free markets. The first to combine a think-tank with a VC, Blue Capital both writes and invests in topical sectors that include fusion power, sustainable energy, and job-positive artificial intelligence. Blue Capital’s strategy is oriented to achieving attractive financial returns whilst increasing the use of responsible power.
Web: www.aeblue.com
Logo – https://mma.prnewswire.com/media/2590942/blue_capital_1_Logo.jpg
![]() View original content:https://www.prnewswire.co.uk/news-releases/oxford-don-levys-vc-think-tank-blue-capital-announces-hon-hugh-e-powell-as-partner-302342703.html
View original content:https://www.prnewswire.co.uk/news-releases/oxford-don-levys-vc-think-tank-blue-capital-announces-hon-hugh-e-powell-as-partner-302342703.html

Fintech PR
Zoomlion Accelerates Global Expansion with Localized Innovations in Saudi Arabia
RIYADH, Saudi Arabia, Jan. 6, 2025 /PRNewswire/ — Zoomlion Heavy Industry Science & Technology Co., Ltd. (“Zoomlion”, 1157.HK) successfully hosted a key account networking and technology launch event on December 19 in Riyadh, Saudi Arabia, unveiling 24 localized innovative products and several intelligent construction solutions. The event showcased Zoomlion’s commitment to advancing its globalization strategy and strengthening partnerships in the Saudi market.
The event featured the launch of 24 products across seven major construction equipment categories, including mobile cranes, tower cranes, concrete, earthmoving, aerial work platforms, industrial vehicles and more. Alongside these innovations, the company introduced three intelligent solutions tailored for mining, nuclear plants, and infrastructure projects, as well as five core intelligent systems.
At the event, Zhan Chunxin, Chairman and CEO of Zoomlion, engaged with clients on product quality, service efficiency, and spare parts support. He also visited local construction project sites, connecting closely with local clients. His visit reinforced the company’s dedication to deepening collaboration and meeting the evolving needs of the Saudi market.
Zoomlion has designed its products with precise adaptability according to the environment and working conditions to meet local needs. The fully tested and verified products also take the Saudi drivers’ control routine habits into consideration to elevate the operating experience.
Clients expressed their confidence in Zoomlion’s capabilities, with Shawaf, Vice President of SHAWAF Company, highlighting their decade-long partnership as a testament to the mutual trust and success shared by both parties. He stated that the event was a great opportunity to connect with more industry peers and he hopes SHAWAF and Zoomlion can further strengthen their cooperation and achieve win-win success.
Zoomlion has been a key player in the Saudi market since entering the region in 2006. In 2021, the company established a local subsidiary and developed a comprehensive service network comprising 16 branches under its “1+11+4” structure. This framework enables full-coverage services, timely technical support, and efficient spare parts supply, further solidifying its reputation as a trusted partner in the region. Over the years, Zoomlion’s strategic presence has contributed to its sustained growth and strengthened its relationships with clients.
The Saudi market serves as a pivotal region for Zoomlion’s global strategy and a critical platform for advancing localized technology and achieving mutual growth. Through continued innovation in products and services, Zoomlion aims to deliver superior experiences to customers in Saudi Arabia and neighboring regions, reinforcing its competitive edge.
View original content:https://www.prnewswire.co.uk/news-releases/zoomlion-accelerates-global-expansion-with-localized-innovations-in-saudi-arabia-302342704.html

Fintech PR
Hyundai Motor Group Executive Chair Euisun Chung Outlines 2025 Vision Driven by Commitment to Innovation, Overcoming Challenges, and Creating Opportunities in New Year’s Message

- Hyundai Motor Group celebrates New Year at Hyundai Motorstudio Goyang, emphasizing open communication with employees at flagship space
… Executive Chair Euisun Chung highlights the need to face challenges head on at 2025 New Year’s Remarks, strengthening the Group’s vision
… Key HMG executives held ’roundtable’, discussing the future global business environment and the Group’s vision following questions from employees - Executive Chair Chung underscores the need for perspective in facing difficulty, emphasizing innovation and overcoming challenges is part of the Group’s DNA
… Preparation and fundamental capabilities are key Hyundai Motor Group assets to help navigate and embrace change
… Agile responses to unexpected challenges through flexible processes and a culture of unified action to achieve collective goals - Open culture where talent can thrive and teamwork is further strengthened to underscore the Group’s resilience and focus on opportunities for growth
… Ensuring an environment where creative and enthusiastic individuals can demonstrate their capabilities to the fullest
… Investment in key business areas and strategic collaboration with partners
SEOUL, South Korea, Jan. 6, 2025 /PRNewswire/ — Hyundai Motor Group (the Group) today held its annual New Year’s Address, with Executive Chair Euisun Chung outlining the Group’s focus for 2025: overcoming challenges through a commitment to innovation, embracing change, and further strengthening teamwork.
Held at Hyundai Motorstudio Goyang, near Seoul, this year’s event adopted a new format to drive in-depth dialogue among attendees, discussing the global business environment for the year ahead and the Group’s strategic direction.
Executive Chair Chung began by thanking Hyundai Motor Group employees around the world. “We achieved a lot last year,” he said. “These achievements were the result of your tireless efforts to deliver the quality, trust and experience our customers expect. I extend my deepest gratitude to you all.”
He continued by emphasizing the importance of facing both internal and external challenges over the year ahead, as well as the potential for global growth across the Group’s operations by overcoming adversity through its commitment to innovation, further strengthening teamwork, and a proactive approach to creating future opportunities.
“Innovation is in Hyundai Motor Group’s DNA. If we continue to embrace change and pursue innovation, we can overcome any test or difficulty we may face,” said Executive Chair Chung.
Executive Chair Chung also reinforced Hyundai Motor Group’s resilience and its ability to further strengthen its position as a global mobility leader by looking for opportunities in every challenge.
“There is no need to be intimidated by uncertainties ahead. Without challenges, we risk becoming complacent, which presents a bigger danger. We cannot assume success in 2025 simply because of our strong performance last year. But we should also not be pessimistic as a defensive mindset can stifle innovation. Challenges can sharpen awareness and drive action – Hyundai Motor Group has successfully navigated challenges in the past and emerged stronger. We will do the same again.”
Addressing challenges and creating opportunities
Executive Chair Chung categorized two types of challenges the Group is facing – ‘predictable’ and ‘unexpected’ – and outlined strategies for overcoming both.
He stressed that thorough preparation is key in overcoming predictable challenges, adding that “It’s not simply about eliminating risks, but about a comprehensive understanding of the background, context, and historical trends to create opportunities for future growth.”
Chung continued by highlighting the importance of fundamental capabilities as a key factor in addressing unexpected challenges, including flexible and open processes, a culture of objective analysis and agile response, and continuous, unified action to achieve the Group’s shared goals.
With the appointment of José Muñoz as Hyundai Motor’s first non-Korean CEO, Executive Chair Chung emphasized this milestone as “a clear expression of our commitment to innovation”, reiterating the Group’s dedication to creating a global culture where talented individuals are recognized and can thrive regardless of their nationality, gender, seniority or background.
Executive Chair Chung closed the Group’s 2025 New Year’s Address by highlighting that “We must expand our strong commitment to innovation,” linking leadership in industrial change and technological development, strategic investment in core areas for the Group, and collaboration with other partners when necessary.
“Our greatest asset is our people. Their talent and resilience mean we do not retreat when we face adversity – we innovate,” said Executive Chair Chung, closing the roundtable. “We embrace challenges as opportunities to grow stronger and to shape a brighter, more sustainable future. We will continue to work together in 2025 to further strengthen Hyundai Motor Group’s collective vision.”
More information about Hyundai Motor Group can be found at: http://www.hyundaimotorgroup.com
Photo – https://mma.prnewswire.com/media/2591023/Photo_1__HMG_New_Year_s_Message_2025.jpg
Photo – https://mma.prnewswire.com/media/2591024/Photo_2__HMG_New_Year_s_Message_2025.jpg
Photo – https://mma.prnewswire.com/media/2591025/Photo_3__HMG_New_Year_s_Message_2025.jpg
PDF – https://mma.prnewswire.com/media/2591026/250106__Press_Release__HMG_New_Year_s_Message_2025.pdf
![]() View original content to download multimedia:https://www.prnewswire.co.uk/news-releases/hyundai-motor-group-executive-chair-euisun-chung-outlines-2025-vision-driven-by-commitment-to-innovation-overcoming-challenges-and-creating-opportunities-in-new-years-message-302342767.html
View original content to download multimedia:https://www.prnewswire.co.uk/news-releases/hyundai-motor-group-executive-chair-euisun-chung-outlines-2025-vision-driven-by-commitment-to-innovation-overcoming-challenges-and-creating-opportunities-in-new-years-message-302342767.html

-

 Fintech PR6 days ago
Fintech PR6 days agoOutsourced Accounting Service: The New Standard for Business Finance Industry
-

 Fintech PR6 days ago
Fintech PR6 days agoBybit Launchpad Onboards Xterio, Opening up Opportunities in Blockchain Gaming for Users
-

 Fintech PR6 days ago
Fintech PR6 days agoCKGSB Professor Mei Jianping Launches Global Indices Tracking Impressionist, Contemporary, and Chinese Art Markets
-

 Fintech PR6 days ago
Fintech PR6 days agoIBN Technologies Sets the Benchmark in Financial Management Accounting Excellence
-

 Fintech PR7 days ago
Fintech PR7 days agoIBN Technologies LLC Steps in to Support Clients After Bench Accounting’s Unexpected Closure
-
 Fintech PR6 days ago
Fintech PR6 days agoDayOne Launches as an Independent Global Data Center Pioneer Following Series B Funding Closure
-

 Fintech PR3 days ago
Fintech PR3 days agoBybit x Block Scholes Report: BTC Options Steady with Call-Put Parity, ETH Braces for Short-Term Volatility
-

 Fintech PR6 days ago
Fintech PR6 days agoGES Completes Sale to Truelink Capital