Fintech PR
GemVax Announces Topline Results from Phase 2a Progressive Supranuclear Palsy Clinical Trial at Neuro2024

– Topline supports moving to Phase 3 trial and shows potential to develop GV1001 as the world’s first PSP treatment
SEOUL, South Korea, Oct. 29, 2024 /PRNewswire/ — GemVax & KAEL Co., Ltd. (“GemVax”; KOSDAQ ticker: 082270) announced that topline results of a Phase 2a clinical trial (the “Phase 2a PSP Clinical Trial”) of GV1001, an investigational peptide drug for the treatment of progressive supranuclear palsy (“PSP”), were presented at “Neuro2024: The PSP and CBD International Research Symposium” in Toronto, Canada, at 4:45 p.m. local time on 24th October.
PSP is a degenerative disease that, like Parkinson’s disease, causes symptoms such as gait disturbances, early falls, vertical gaze palsy, rigidity, tremors, and cognitive decline, but it progresses faster and currently has no fundamental treatment. PSP is classified into several types, including PSP-Richardson’s syndrome (“PSP-RS”) and PSP-parkinsonism (“PSP-P”). Compared to other types of PSP, the PSP-RS type shows a greater accumulation of tau protein and affects larger areas, including the cerebellum, dentate nucleus, pontine nuclei, frontal lobe, and parietal lobe.
The Phase 2a PSP Clinical Trial was a 24-week, randomized, double-blind, placebo-controlled, prospective exploratory clinical trial conducted in 78 patients with PSP at 5 centers in Korea. The participants were randomized 1:1:1 to receive either placebo or GV1001 0.56 mg or GV1001 1.12 mg administered subcutaneously once weekly for the first 4 weeks (1 month), and then at 2-week intervals for 20 weeks (5 months) for a total of 24 weeks (6 months). Patients with both PSP-RS and PSP-P types were eligible to participate in the study. Results showed higher benefits in the lower dose group (0.56 mg), particularly in PSP-RS type patients.
The primary endpoint of the trial was change from baseline in total score (calculated as the least-square mean using MMRM method) of PSP-Rating Scale after 24 weeks of GV1001 administration, which showed deterioration by 2.14 points in GV1001 0.56 mg dose group compared to 4.10 points in the placebo group, demonstrating a 48% reduction in disease progression (see Figure 1). Although statistical significance was not demonstrated, the results support the potential of GV1001 as a treatment of PSP, a disease for which there is currently no cure, and the potential to advance GV1001 into further clinical trials.
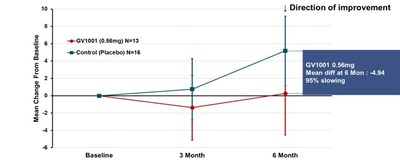
The clinically typical PSP is often referred to as the PSP-RS type, which accounts for the majority of PSP patients. This type progresses faster and has a shorter average survival time compared to other PSP types. Subgroup analysis was conducted in patients with PSP-RS type only. The change from baseline in PSP-Rating Scale total score mean (calculated using simple average) at 24 weeks of GV1001 administration to PSP-RS type patients was a deterioration by 0.25 points in the GV1001 0.56 mg dose group compared to a deterioration by 5.19 points in the placebo group, demonstrating a 4.94-point difference or a 95% reduction in disease progression (see Figure 2).
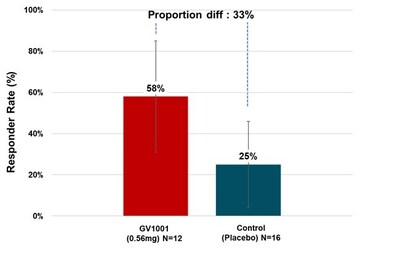
Many PSP-RS type patients in the treatment group experienced symptom stabilization or even improvement during the clinical period. When calculated as responder rate based on the percentage of patients whose PSP Rating Scale scores improved or remained stable after six months of treatment compared to baseline, 58.33% of PSP-RS type patients in the 0.56 mg GV1001 group showed improvement or stabilization (see Figure3).
The safety profile of GV1001 in the Phase 2a PSP Clinical Trial was consistent with prior safety data. GV1001 was generally well-tolerated with no serious adverse events related to the drug reported.
Hyungsik Moon, CSO of GemVax, stated that “this Phase 2a trial was an exploratory study to determine the optimal dosage and find out how the peptide works on different subgroups. Although the topline result did not achieve statistical significance, the evidence is strong enough to consider moving forward to a pivotal trial and shows potential to develop GV1001 as the world’s first treatment option for PSP.”
Experts at the Neuro2024 meeting welcomed the results of the PSP trial as encouraging and expressed excitement for the drug to enter a global Phase 3 clinical trial for further development.
“This pilot study was not fully powered and the treatment duration with 6 months was short. Thus, statistically significant confirmatory results could not be expected” said Peter Schüler, MD, Senior Vice President of Drug Development at global CRO ICON. “Nonetheless, the observed trends are very plausible and consistent in two domains: motor performance and cognitive function, both favoring the lower dose group.”
“The trial identified the optimal dose, which was one of the primary objectives of the Phase 2a study, and demonstrated clinically meaningful benefits, namely full stabilization of the disease compared to the placebo group,” said Dr. Schüler, adding “these topline results provide a strong foundation for advancing to Phase 3.”
Dr. Günter U. Höglinger, Head of the Department of Neurology, LMU Hospital, Munich, and a world-renowned expert in PSP, commented: “very exciting Phase 2 level data with novel drug study with new mechanisms of action. Data is preliminary but very promising and it is in line with [GV1001] Alzheimer’s disease clinical data. I look forward to further development and very excited to participate and lead the [PSP] Phase 3 study.”
Dr. Kristophe Diaz, Director of CurePSP, said that “we are encouraged by the results of the recent GemVax clinical trial, which offer hope to the entire PSP community, including patients who currently have no treatment options, their families and the physicians who care for them” and that “we congratulate GemVax on the successful completion of this trial and look forward to further developments that benefit the PSP community.” He also said that “CurePSP remains committed to collaborating and supporting efforts that bring hope and progress for those affected by this devastating disease.”
Download PDF: https://mma.prnewswire.com/media/2542940/2024_10_29__GemVax_Announces_Topline_Results.pdf
About Phase 2a PSP Clinical Trial (NCT05819658)
The Phase 2a PSP clinical trial was a 24-week, multicenter, randomized, double-blind, placebo-controlled, prospective phase 2a exploratory clinical trial to evaluate the safety and efficacy of GV1001 0.56 mg or 1.12 mg compared to placebo for the treatment of patients with PSP. The primary outcome of the study was change from baseline in the total score of PSP-Rating Scale after 24 weeks of GV1001 administration. Secondary endpoints included change from baseline in the total score of PSP-Rating Scale at 3 months, MoCA-K, K-FAB and ES-ADL at both 3 and 6 months. Overall safety of GV1001 administration was also assessed.
About GV1001
GV1001 is a synthetic peptide consisting of 16 amino acids based on the key sequence of telomerase. GV1001 has been studied for the potential treatment of neurodegenerative diseases including Alzheimer’s disease and PSP. In neurodegenerative diseases, GV1001 has been demonstrated to modulate phenotypes of glial cells, and to regulate neuroinflammation. In addition to the Phase 2a PSP clinical trial, a Phase 2 Alzheimer’s disease clinical trial of GV1001 is currently ongoing in the U.S. and Europe (NCT05189210).
About PSP
Progressive supranuclear palsy is a rare progressive and adult-onset neurodegenerative disease that currently has no disease-modifying drug. Approximately seven in 100,000 people worldwide is affected by PSP and is more common in men. People over the age of 60 are mainly affected. The symptoms of PSP include loss of balance, changes in personality, weakness of eye movements, especially in the downward direction, difficulty in swallowing, slurred speech and cognitive impairment.
About GemVax & KAEL
GemVax & KAEL Co., Ltd. is a pioneering clinical-stage biopharmaceutical company based in Korea, dedicated to developing proprietary therapeutics for neurodegenerative diseases including progressive supranuclear palsy and Alzheimer’s disease. As for PSP, GemVax is currently conducting a Phase 2a study in Korea to evaluate the efficacy and safety of GV1001 in patients with PSP. Preparations are also underway for a global PSP clinical trial. In addition, GemVax is currently conducting a Phase 2 Alzheimer’s disease clinical trial in the U.S. and Europe. For more information, visit www.gemvax.com and follow us on Linkedin.
Forward-Looking Statements
This document contains information that includes or is based upon “forward-looking statements” within the meaning of the Securities Litigation Reform Act of 1995. Forward-looking statements may or may not include identifying words such as “plan,” “will,” “expect,” “anticipate,” “intend,” “believe,” “potential,” “continue,” and similar terms. These statements are subject to known or unknown risks and uncertainties that could cause actual results to differ materially from those expressed or implied in such statements, including but not limited to: challenges inherent in pharmaceutical research and development, including the timing and results of preclinical and clinical programs, where the risk of failure is high and failure can occur at any stage prior to or after regulatory approval due to lack of sufficient efficacy, safety considerations, or other factors; our ability to leverage and enhance our drug discovery platform; our ability to obtain financing for development activities and other corporate purposes; the success of our collaboration activities; our ability to obtain regulatory approval of, and ultimately commercialize, drug candidates; our ability to obtain, maintain, and enforce intellectual property protections; cyberattacks or other disruptions to our technology systems; our ability to attract, motivate, and retain key employees and manage our growth; inflation and other macroeconomic issues; and other risks and uncertainties. All forward-looking statements are based on management’s current estimates, projections, and assumptions, and GemVax undertakes no obligation to correct or update any such statements, whether as a result of new information, future developments, or otherwise, except to the extent required by applicable law.
Photo – https://mma.prnewswire.com/media/2543033/Figure_1.jpg
Photo – https://mma.prnewswire.com/media/2543034/Figure_2.jpg
Photo – https://mma.prnewswire.com/media/2543035/Figure_3.jpg
PDF – https://mma.prnewswire.com/media/2543036/2024_10_29__GemVax_Announces_Topline_Results.pdf
Logo – https://mma.prnewswire.com/media/2542900/4994690/GemVax_Logo.jpg
![]() View original content to download multimedia:https://www.prnewswire.co.uk/news-releases/gemvax-announces-topline-results-from-phase-2a-progressive-supranuclear-palsy-clinical-trial-at-neuro2024-302289880.html
View original content to download multimedia:https://www.prnewswire.co.uk/news-releases/gemvax-announces-topline-results-from-phase-2a-progressive-supranuclear-palsy-clinical-trial-at-neuro2024-302289880.html

Fintech PR
PostEra announces expansion to $610M in their AI drug discovery collaboration with Pfizer
BOSTON, Jan. 7, 2025 /PRNewswire/ — PostEra, a biotechnology company specializing in machine learning for preclinical drug discovery, today announced an expansion of their partnership with Pfizer. The parties will launch a new Antibody-Drug-Conjugate (ADC) collaboration while also expanding their existing $260M AI Lab collaboration, which itself was built upon a successful Generative Chemistry partnership.
The teams will leverage PostEra’s AI platform, Proton, a pioneering innovation in generative chemistry and synthesis-aware design, to advance several programs. These new programs include small molecule therapeutics as well as ADCs, where PostEra will use Proton to optimize properties of payloads.
PostEra will receive an upfront payment of $12M and is eligible to receive additional milestone payments and tiered royalties on any approved products arising out of the collaboration.
Over the last 3 years, as part of the AI Lab, PostEra and Pfizer scientists have partnered closely to advance several small molecule programs. After Pfizer nominated the maximum number of programs, the teams have agreed to expand the collaboration to include additional targets with PostEra receiving additional upfront payment and eligibility for milestones and royalties.
“We’re pleased to significantly expand the use of PostEra’s Proton platform. This builds on peer-reviewed publications with Pfizer validating the real-world impact of AI-driven drug discovery in hitting preclinical milestones faster than anticipated,” said Alpha Lee, Chief Scientific Officer of PostEra. “This third partnership with our long-term collaborators at Pfizer underscores Proton’s depth and strength in making a meaningful impact on real-world drug discovery campaigns,” added Aaron Morris, CEO of PostEra.
About PostEra
PostEra is building a modern 21st century biopharma. We use Proton, our AI platform for medicinal chemistry, to accelerate the discovery of new medicines for patients. PostEra is advancing an internal pipeline while also advancing small molecule programs through partnerships with biopharma. We’ve closed over $1Bn in AI partnerships including 4 multi-year agreements with Pfizer and Amgen. PostEra is also leading an antiviral drug discovery center for pandemic preparedness, funded by one of the largest grants in NIH history.
Logo – https://mma.prnewswire.com/media/1722598/PostEra_Logo.jpg
![]() View original content:https://www.prnewswire.co.uk/news-releases/postera-announces-expansion-to-610m-in-their-ai-drug-discovery-collaboration-with-pfizer-302342058.html
View original content:https://www.prnewswire.co.uk/news-releases/postera-announces-expansion-to-610m-in-their-ai-drug-discovery-collaboration-with-pfizer-302342058.html

Fintech PR
Radius Global Market Research Acquires Illuminas North America

The partnership combines Radius’s strategic insights framework with Illuminas’s expertise in the technology and financial services sectors, thereby strengthening Radius’s capabilities across B2B and consumer markets.
NEW YORK, Jan. 7, 2025 /PRNewswire/ — Radius Global Market Research, a leading global insights and strategy firm, has announced its acquisition of Illuminas North America, a multidisciplinary research consultancy with headquarters in Austin, Texas. The acquisition strengthens Radius’s ability to deliver actionable insights for its global clients and enhances its expertise in supporting strategic insights needs of clients across industries.
Financial details were not disclosed.
Combining Expertise for Greater Insights
The acquisition integrates Radius’s Brand Growth Navigator framework with Illuminas’s strength in data science and deep expertise with technology and service-related industries. Illuminas is renowned for bridging gaps in customer understanding through tailored, data-driven solutions that illuminate optimal paths to success and drive growth for global brands.
“Illuminas’s proven capabilities in technology-focused research and their expertise in supporting B2B companies make them an ideal partner for Radius,” said Chip Lister, managing director of Radius Global Market Research. “This partnership enhances our ability to deliver insights that address critical business challenges for our clients, especially in industries where technology and innovation are key drivers of success.”
Expanding Capabilities for Clients Worldwide
Founded in 2002, Illuminas North America has built a reputation as a trusted partner for Fortune 500 companies and industry leaders. With deep expertise in technology, financial services, and dynamic global markets, Illuminas employs innovative and foundational research techniques, including quantitative and qualitative tools, to deliver insights that go beyond data to uncover compelling narratives.
“Our partnership with Radius will allow us to expand the reach and impact of our work,” said Jay Shutter, Principal and CEO of Illuminas. “By combining our customer-focused methodologies with Radius’s strategic insights framework, we’ll be better equipped to deliver actionable research that empowers our clients to make confident, informed decisions. This is a tremendous opportunity to enrich the value we provide to clients across the globe.”
Global Reach and Local Expertise
Illuminas North America’s offices in Austin, Texas, and Great Falls, Virginia, will enhance Radius’s ability to deliver insights worldwide. This acquisition follows Radius’s January 2025 acquisition of 7th Sense and its January 2024 acquisition of London-based Strive Insight, further extending the firm’s global footprint. Together, Radius and Illuminas will provide a seamless integration of advanced research tools and industry-specific expertise to support clients in achieving their goals.
About Radius Global Market Research
Founded in 1960, Radius is a full-service marketing research consultancy headquartered in New York City, with offices across the U.S. and globally. Radius supports brand growth through its Brand Growth Navigator framework, helping clients align insights with strategic priorities to maximize ROI. Its expertise spans industries, including technology, financial services, and consumer goods. Visit www.radiusinsights.com for more information.
About Illuminas North America
Illuminas is a strategic market research consultancy founded in 2002, specializing in bridging gaps in customer understanding. Headquartered in Austin, Texas, with an office in Great Falls, Virginia, Illuminas provides customized research solutions using proprietary methodologies to uncover insights for technology, financial services, and hospitality industries. The team combines quantitative and qualitative research methods to deliver insights that empower decision-making and drive business growth. Visit www.us.Illuminas.com for more information.
Logo – https://mma.prnewswire.com/media/2223227/Radius_logo_Logo.jpg
![]() View original content:https://www.prnewswire.co.uk/news-releases/radius-global-market-research-acquires-illuminas-north-america-302344596.html
View original content:https://www.prnewswire.co.uk/news-releases/radius-global-market-research-acquires-illuminas-north-america-302344596.html

Fintech PR
Fisher Investments Finalizes Strategic Partnership with Advent and ADIA with Completion of Minority Common Stock Investment
Fisher Investments’ Founder Ken Fisher Maintains Majority Controlling Interest
PLANO, Texas, Jan. 7, 2025 /PRNewswire/ — Fisher Investments (“FI”) announced that Advent International (“Advent”) and a wholly owned subsidiary of the Abu Dhabi Investment Authority (“ADIA”) completed a previously announced minority investment in Ken Fisher’s namesake firm, Fisher Investments. The $3 billion common stock investment by Advent and ADIA values FI at $12.75 billion.
The transaction was part of Ken Fisher’s long-term estate planning and ensures FI’s long-term private independence, culture, growth evolution and devotion to exceptional client service. Ken Fisher remains active in his current role as FI’s Executive Chairman and Co-Chief Investment Officer and retains a majority of beneficial ownership and over 70% of voting shares in FI. FI CEO Damian Ornani continues to drive FI’s day-to-day operations and business strategy. In connection with the investment, David Mussafer, a Managing Partner at Advent, has joined the board of directors at FI, and Gabriela Weiss, a Principal at Advent, has joined as a board observer at FI.
As of 12/31/24, FI managed nearly $300 billion for over 170,000 clients globally, including over 130,000 US private clients and 200 of the world’s largest and most well-known institutional clients. This is the first outside investment in FI, with previous ownership solely among family and employees. There is no further FI investment transaction contemplated. The investment in common shares includes neither options nor non-common stock preferences and includes proportional voting to the investors’ beneficial ownership in FI.
Ken Fisher said, “While my health is excellent, this transaction is aimed dually at long-term estate tax and planning purposes should anything untoward happen to me. Advent and ADIA are truly exceptional partners who value us operationally and culturally, and are committed to preserving what differentiates FI in our industry.”
Damian Ornani, longtime FI CEO, said, “We welcome Advent and ADIA’s support of our mission to help more new clients around the world.”
David Mussafer said, “We are thrilled to cement Advent’s partnership with FI at a moment when there is a growing need for the smart, independent and personalized financial expertise that FI is recognized for providing for 45 years. We look forward to closely collaborating with Ken, Damian and the rest of the FI team to support the company’s continued growth, drawing on Advent’s deep expertise in helping financial services companies best capitalize on the opportunities ahead.”
J.P. Morgan Securities LLC and RBC Capital Markets served as joint financial advisors and Paul Hastings served as legal advisor to FI. Ropes & Gray served as legal advisor to Advent. Gibson Dunn served as legal advisor to ADIA.
About Fisher Investments
Founded in 1979, Fisher Investments is an independent, fee-only investment adviser. Fisher Investments and its subsidiaries manage nearly $300 billion across three principal businesses—Institutional, US Private Client, and Private Client International. Founder and Executive Chairman Ken Fisher wrote the Forbes “Portfolio Strategy” column for 32 ½ years until 2017, making him the longest running columnist in its history. He now writes monthly for the New York Post and discreet unique columns in native language, varying by country, in 26 major nations, spanning more countries and more total volume than any other columnist of any type in history. Ken has appeared regularly on major TV news like Fox Business and News, BBN Bloomberg and CNN International. Ken has written 11 investing and finance books, including four New York Times bestsellers. For more information, visit www.fisherinvestments.com.
About Advent International
Advent is a leading global private equity investor committed to working in partnership with management teams, entrepreneurs, and founders to help transform businesses. With 16 offices across five continents, we oversee more than USD $88.8 billion in assets under management* and have made more than 420 investments across 43 countries.
Since our founding in 1984, we have developed specialist market expertise across our five core sectors: business & financial services, consumer, healthcare, industrial, and technology. This approach is bolstered by our deep sub-sector knowledge, which informs every aspect of our investment strategy, from sourcing opportunities to working in partnership with management to execute value creation plans. We bring hands-on operational expertise to enhance and accelerate businesses.
As one of the largest privately-owned partnerships, our 650+ colleagues leverage the full ecosystem of Advent’s global resources, including our Portfolio Support Group, insights provided by industry expert Operating Partners and Operations Advisors, as well as bespoke tools to support and guide our portfolio companies as they seek to achieve their strategic goals.
To learn more, visit our website connect with us on LinkedIn.
*Advent assets under management (AUM) as of June 30, 2024. AUM includes assets attributable to Advent advisory clients as well as employee and third-party co-investment vehicles.
About Abu Dhabi Investment Authority
Established in 1976, the Abu Dhabi Investment Authority (“ADIA”) is a globally diversified investment institution that prudently invests funds on behalf of the Government of Abu Dhabi through a strategy focused on long-term value creation. For more information, visit www.adia.ae.
Media Contacts
For Fisher Investments
Naj Srinivas
Executive Vice President, Corporate Communications
[email protected]
For Advent International
Leslie Shribman
Head of Communications
[email protected]
For ADIA
Garry Nickson
Corporate Communications & Public Affairs
[email protected]
Logo – https://mma.prnewswire.com/media/2592020/Fisher_Investments_Logo.jpg
![]() View original content:https://www.prnewswire.co.uk/news-releases/fisher-investments-finalizes-strategic-partnership-with-advent-and-adia-with-completion-of-minority-common-stock-investment-302344504.html
View original content:https://www.prnewswire.co.uk/news-releases/fisher-investments-finalizes-strategic-partnership-with-advent-and-adia-with-completion-of-minority-common-stock-investment-302344504.html

-

 Fintech PR4 days ago
Fintech PR4 days agoBybit x Block Scholes Report: BTC Options Steady with Call-Put Parity, ETH Braces for Short-Term Volatility
-

 Fintech PR4 days ago
Fintech PR4 days agoArtificial Intelligence (AI) in Trading Market to Reach USD 35 Billion by 2030, Growing at a 10% CAGR | Valuates Reports
-

 Fintech PR5 days ago
Fintech PR5 days agoBookkeeping in USA: Empower Business Growth and Success with IBN Technologies
-

 Fintech PR5 days ago
Fintech PR5 days agoCUBE COMPLETES ACQUISITION OF THOMSON REUTERS REGULATORY INTELLIGENCE AND ODEN BUSINESSES
-

 Fintech PR6 days ago
Fintech PR6 days agoKuCoin Advances the “Menstrual Equity Project”, Benefiting 4,000 Women in the Bahamas
-

 Fintech PR5 days ago
Fintech PR5 days agoDataLend: 2024 Securities Lending Revenue Down 10% YoY to $9.64 Billion
-

 Fintech PR6 days ago
Fintech PR6 days agoKuCoin Launches KuCoin Pay, a Merchant Solution Leading the Future of Crypto Payments
-

 Fintech PR6 days ago
Fintech PR6 days agoInaugural PHBS-IER Conference Highlights Cutting-Edge Economic Research